Abstract
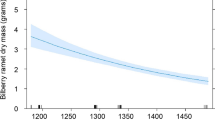
The coastal salt marshes of the Aransas National Wildlife Refuge (ANWR), Texas, USA support a wintering population of the endangered Whooping Crane (Grus americana). Although the bulk of their winter diet is comprised of blue crabs, berries from the Carolina wolfberry (Lycium carolinianum) can contribute 21–52% of crane energy intake early in the wintering period. Monthly, from November 2003 to February 2005, we tracked L. carolinianum growth in nine 1-m2 permanent macrophyte plots along the estuarine gradient to understand the spatial and temporal variability of this perennial halophyte. Lycium carolinianum showed strong seasonal growth patterns, with leaf production peaks in late winter and again in late summer, just prior to flowering, but little significant spatial variation. Flowering of L. carolinianum occurred in October and November, and peak berry abundance coincided with the arrival of the cranes in late October and early November. During this period, the total number of flowers per plant and total number of leaves per plant across all sites were positively related to surfacewater depth and pore-water salinity. The numbers of flowers and berries per plant were significantly higher at our lowest elevation site during the 2004 fruiting season. Berries were rarely observed in the marshes for the remainder of each calendar year. Stem diameter was the best estimator of L. carolinianum aboveground biomass in ANWR marshes, accounting for approximately 94% of the variability (p < 0.001). Monthly changes in estimated aboveground biomass at each site revealed no distinguishable spatio-temporal trends. This is likely a result of L. carolinianum’s woody stem, which accounts for much of the total plant biomass but is much less dynamic than photosynthetic and reproductive tissues.
Similar content being viewed by others
Literature Cited
Alexander, H. D. and K. H. Dunton. 2002. Freshwater inundation effects on emergent vegetation of a hypersaline salt marsh. Estuaries 25: 1426–1435.
Butzler, R. E. 2006. Spatial and Temporal Patterns of Lycium carolinianum Walt., the Carolina Wolfberry, in the Salt Marshes of Aransas National Wildlife Refuge, Texas. M.S. Thesis. Texas A&M University, College Station, TX, USA.
Chavez-Ramirez, F. 1996. Food availability, foraging ecology, and energetics of Whooping Cranes wintering in Texas. Ph.D. Dissertation. Texas A&M University, College Station, TX, es.
Copeland, B. J. 1966. Effects of decreased river flow and fauna on estuarine ecology. Journal Water Pollution Control Federation 38: 1831–1839.
Daoust, R. J. and D. L. Childers. 1998. Quantifying aboveground biomass and estimating net aboveground primary production for wetland macrophytes using a non-destructive phenometric technique. Aquatic Botany 62: 115–133.
Deegan, L. A., J. Day, J. Gossleink, A. Yàñez-Arancibia, G. Soberòn Chàvez, and P. Sànchez-Gil. 1986. Relationships among physical characteristics, vegetation distribution and fisheries yield in Gulf of Mexico estuaries. p. 83–100, In D. Wolfe (ed.) Estuarine Variability. Academic Press, Orlando, FL, USA.
DeLuane, R. D., S. R. Pezeshki, and W. H. PatrickJr. 1987. Response of coastal plants to increase in submergence and salinity. Journal of Coastal Research 3: 535–546.
Dunton, K. H., B. Hardegree, and T. E. Whitledge. 2001. Response of estuarine marsh vegetation to interannual variations in precipitation. Estuaries 24: 851–861.
Fejes, E., D. Roelke, G. Gable, J. Heilman, K. McInnes, and D. Zuberer. 2005. Microalgal productivity, community composition, and pelagic food web dynamics in a subtropical, turbid salt marsh isolated from freshwater inflow. Estuaries 28: 96–107.
Fitch, J. A. and N. E. Armstrong. 1982. Prediction of salinities in the Matagorda Bay area. University of Texas at Austin, Center for Research in Water Resources, Austin, Texas, USA. Techni-Technical Report CRWR-200.
Gallagher, J. L. 1975. Effect of an Ammonium Nitrate pulse on the growth and elemental composition if natural stands of Spartina alterniflora and Juncus roemerianus. American Journal of Botany 62: 644–648.
Godfrey, R. K. and J. W. Wooten. 1981. Aquatic and Wetland Plants of the Southeastern United States. University of Georgia Press, Athens, GA, USA.
Gough, L. and J. B. Grace. 1998. Effects of flooding, salinity, and herbivory on coastal plant communities, Louisiana, United States. Oecologia 117: 527–535.
Gratton, C. and R. F. Denno. 2003. Inter-year carryover effects of a nutrient pulse on Spartina plants, herbivores, and natural enemies. Ecology 84: 2692–2707.
Hopkinson, C. S., J. G. Gosselink, and R. T. Parrondo. 1978. Aboveground production of seven marsh plant species in coastal Louisiana. Ecology 59: 760–769.
Howard, R. J. and I. A. Mendelssohn. 2000. Structure and composition of oligohaline marsh plant communities exposed to salinity pulses. Aquatic Botany 68: 143–164.
Jassby, A. D., W. J. Kimmerer, S. G. Monismith, C. Armor, J. E. Cloern, T. M. Powell, J. R. Schubel, and T. J. Vendilinski. 1995. Isohaline position as a habitat indicator for estuarine populations. Ecological Applications 5: 272–289.
Kennish, M. J. 2001. Coastal salt marsh systems in the U.S.: a review of anthropogenic impacts. Journal of Coastal Research 17: 731–748.
Kuhn, N. L. and J. B. Zedler. 1997. Differential effects of salinity and soil saturation on native and exotic plants of a coastal salt marsh. Estuaries 20: 391–403.
Kuhn, N. L. and I. A. Mendelssohn. 1999. Halophyte sustainability and sea level rise: mechanisms of impact and possible solutions. p. 113–126, In H. Leith (ed.) Halophyte Uses in Different Climates. Backhuys Publishers, Leiden, Netherlands.
Loneragan, N. R. and S. E. Bunn. 1999. River flows and estuarine ecosystems: Implications for coastal fisheries from a review and a case study of the Logan River, southeast Queensland. Australian Journal of Ecology 24: 431–440.
McKee, K. L. and I. A. Mendelssohn. 1989. Response of a freshwater marsh plant community to increased salinity and increased water level. Aquatic Botany 34: 301–316.
Montagna, P. A., R. D. Kalke, and C. Ritter. 2002. Effect of restored freshwater inflow on macrofauna and meiofauna in upper Rincon Bayou, Texas, USA. Estuaries 25: 1426–1435.
Morris, J. T. and B. Haskin. A 5-yr record of annual primary production and stand characteristics of Spartina alterniflora. Ecology 71: 2209–2217.
Odum, W. E. 1988. Comparative ecology of tidal freshwater and salt marshes. Annual Review of Ecology and Systematics 19: 147–176.
Pennings, S. C. and R. M. Callaway. 1992. Salt marsh plant zonation: the relative importance of competition and physical factors. Ecology 73: 681–690.
Pezeshki, S. R. and R. D. DeLaune. 1991. A comparitive study of aboveground productivity of dominant U.S. Gulf Coast marsh species. Journal of Vegetation Science 2: 331–338.
Smart, M. R. and J. W. Barko. 1980. Nitrogen nutrition and salinity tolerance of Distichlis spicata and Spartina alterniflora. Ecology 6: 630–638.
Stutzenbaker, C. D. 1999. Aquatic and Wetland Plants of the Western Gulf Coast. Texas Parks and Wildlife Press, Austin, TX, USA.
Sullivan, G. and G. Noe. 2001. Distribution of plant species in coastal wetlands of San Diego County. p. 369–394, In J. Zedler (ed.) Handbook for Restoring Tidal Wetlands, CRC Press, Boca Raton, FL, USA.
Thursby, G. B., M. M. Chintala, D. Stetson, C. Wigand, and D. M. Champlin. 2002. A rapid, non-destructive method for estimating aboveground biomass of salt marsh grasses. Wetlands 22: 626–630.
Zedler, J. B. 1983. Freshwater impacts in normally hypersaline marshes. Estuaries 6: 346–355.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Butzler, R.E., Davis, S.E. Growth patterns of Carolina wolfberry (Lycium carolinianum L.) in the salt marshes of Aransas National Wildlife Refuge, Texas, USA. Wetlands 26, 845–853 (2006). https://doi.org/10.1672/0277-5212(2006)26[845:GPOCWL]2.0.CO;2
Received:
Revised:
Accepted:
Issue Date:
DOI: https://doi.org/10.1672/0277-5212(2006)26[845:GPOCWL]2.0.CO;2